

???????
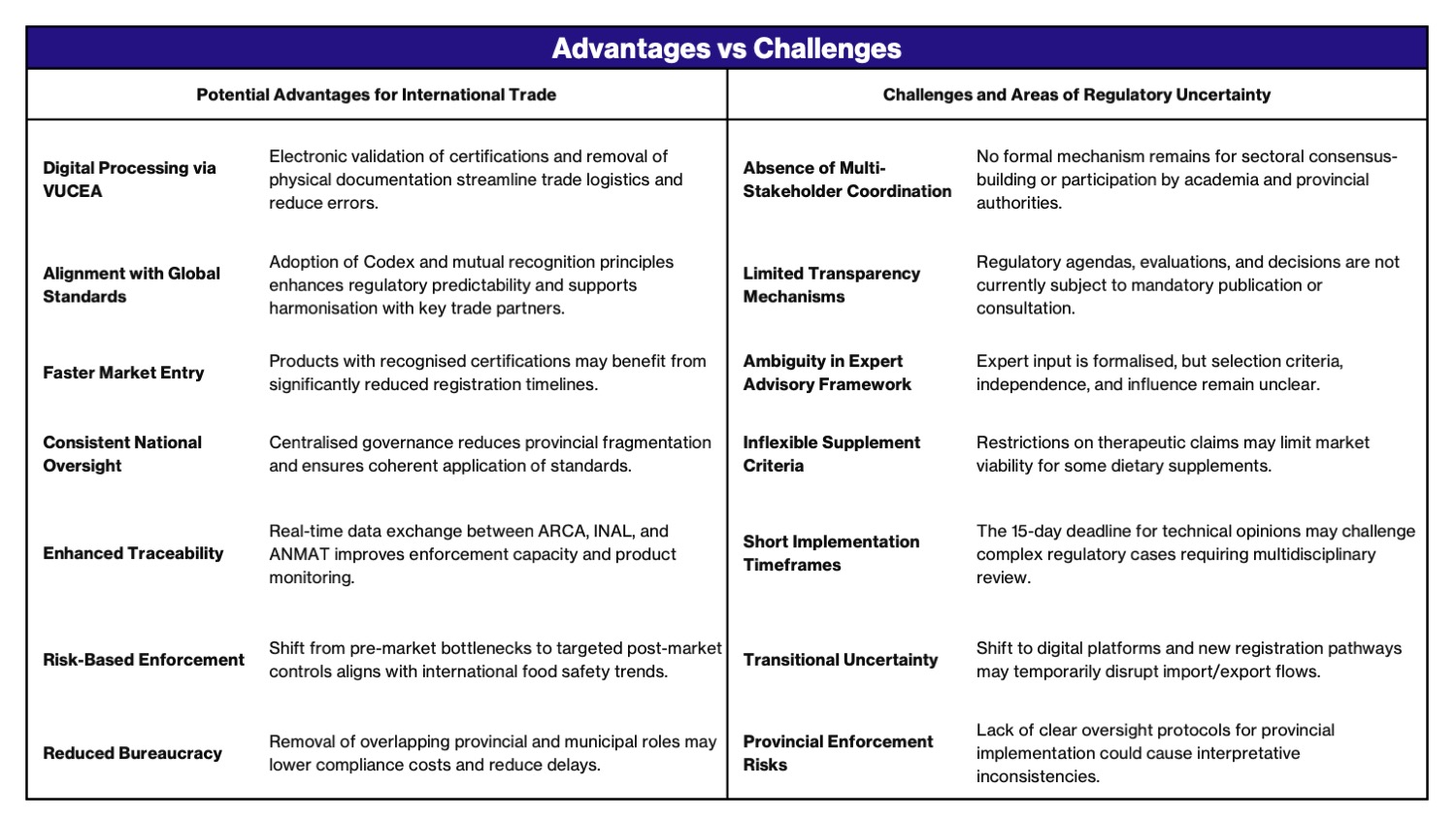
???????????Since January, Argentina has launched a broad transformation of its food regulatory framework through a series of interlinked measures, starting with Decree 35/2025, followed more recently by General Resolution 5731/2025 and Decree 538/2025 . These actions reflect a comprehensive strategy aimed at modernizing food control, aligning with international standards, and enhancing trade facilitation.
These changes emphasize centralized decision-making, digital interoperability, and risk-based surveillance, redefining how food safety is governed and enforced.
Structural and Procedural Changes
- Decision-making on food regulation now resides exclusively with national agencies, ANMAT (for processed foods) and SENASA (for primary agricultural products). Former coordination bodies such as CONAL and CONASE have been dissolved.
- Selected provisions of Decree 815/1999 have been repealed, removing requirements such as municipal authorisations and outdated sanitary barriers, in favour of a national-level model.
- As per General Resolution 5731/2025, the processes for notification, certification, and authorisation of food imports and exports now operate entirely through the Foreign Trade Single Window (VUCEA), enabling electronic validation of Licences, Permits, Certificates, and Other Documents (LPCO). This builds on the digital transition initiated by Decree 35/2025.
- ANMAT now oversees a centralised database consolidating data on establishments, product registrations, sanctions, and inspections.
- Import procedures allow for simplified entry of products from countries recognised for high sanitary oversight (e.g., EU, United States, Canada, Australia), provided supporting documentation such as Free Sale Certificates are submitted.
- International food safety standards have been formally adopted within risk evaluation and post-market surveillance frameworks.
- ARCA and ANMAT?s National Food Institute (INAL) now coordinate operations through VUCEA?s live dashboard, enabling traceability and oversight.
- Expert contributions to regulatory development must be submitted within 15 business days of appointment, establishing accelerated review cycles.
- Products from countries listed in Annex III of Decree 35/2025 are exempt from RNPA numbering but must comply with CAA labelling rules. Dietary supplements making therapeutic claims are prohibited from being marketed. This Decree also has favourable impact on product composition and/or use of food additives.
- Emphasis has shifted towards risk-based controls following product entry, consistent with global best practices in food safety enforcement.
Strategic Considerations for International Trade
- Eligibility for simplified import procedures depends on recognition status, which determines documentation requirements.
- All importers and exporters must operate within the VUCEA system for document submission and validation.
- Certificates of Free Sale, labelling, and technical documentation must comply with CAA and Codex requirements.
- Nutritional products must be clearly distinguished from medicinal products in both formulation and marketing.
- Regular updates from ARCA, ANMAT, SENASA, and INAL will be critical to understanding procedural changes during the transition.
- Internal compliance systems should be adapted to interact with VUCEA and ANMAT?s national database.
- Registered operators and legal representatives with experience in Argentine regulatory processes will be essential in managing transitions.
- Given the limited availability of public records, internal tracking of submissions and approvals is recommended for risk management.
- Labelling strategies, product categories, and origin countries should be reviewed in light of the new requirements and eligibility criteria.
- Depending on product origin and classification, both streamlined and full registration routes may coexist, requiring flexible planning.
Reach out to us if you are interested in exploring opportunities for your products in Argentina and across Latin America.
Published: August 13, 2025
© All rights reserved. All news and diagrams placed on this Web site is made for internal use. Its reproduction or distribution in any form are welcome in case of placing a direct hyperlink to a source. Reproduction or distribution of information which contains FJS International Solutions® as a source is prohibited without the written permission from the FJS International Solutions®. Photos placed on this site are taken from open sources only or purchased.