
On August 11, 2025, the Mexican Secretariat of Health, through the Federal Commission for the Protection against Sanitary Risks (COFEPRIS), has gazetted a new regulatory Agreement aimed at simplifying import procedures for food products and dietary supplements. This measure aligns with the provisions of the National Law for the Elimination of Bureaucratic Procedures and the objectives set forth in the National Development Plan 2025?2030.
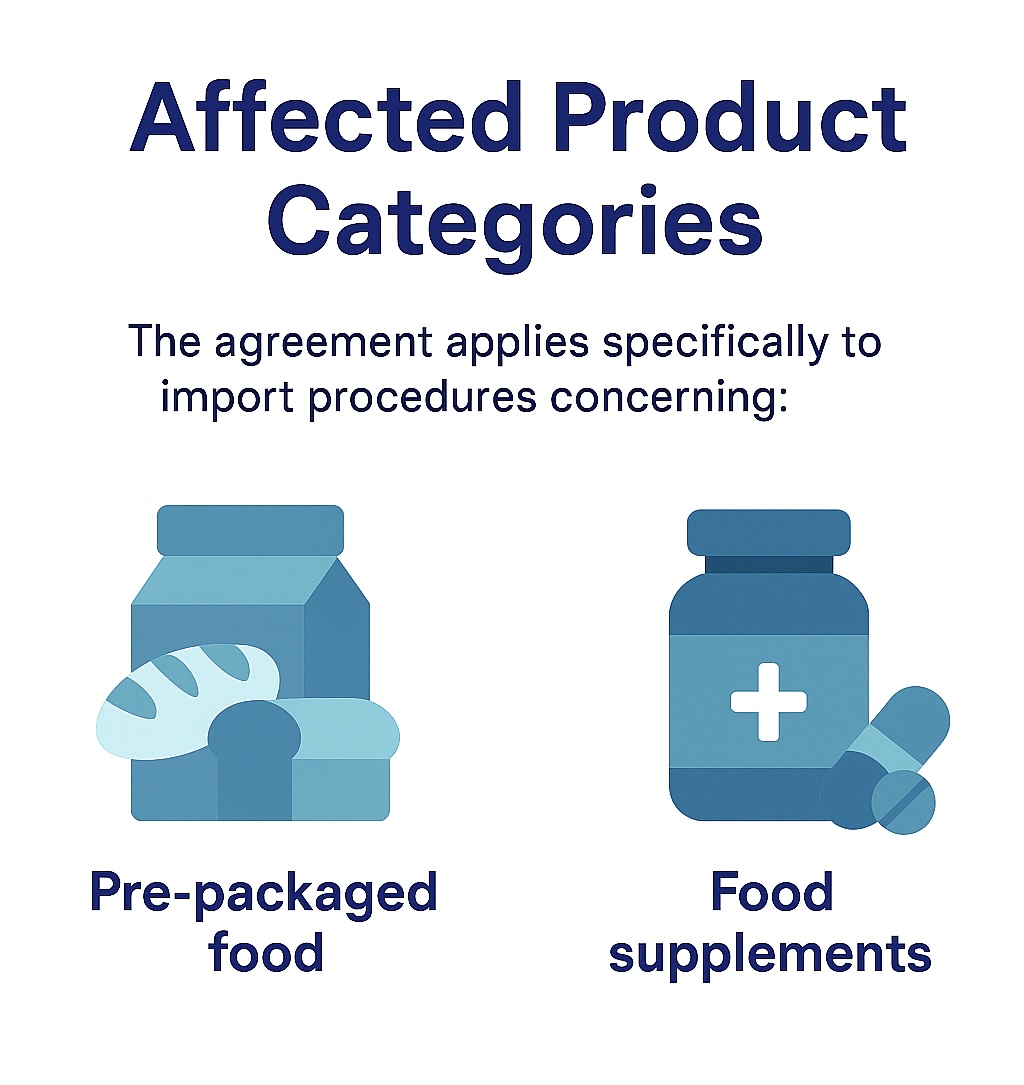
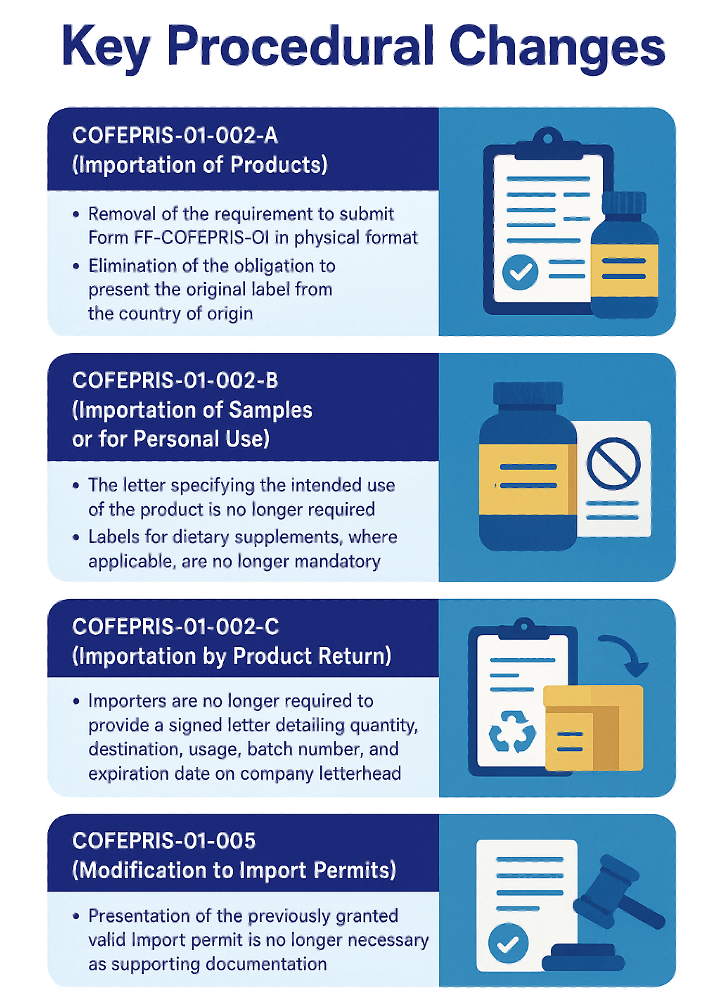
The reform introduces the elimination of specific physical documentation requirements, promotes the use of digital platforms, and reduces administrative burden for importers and institutions engaged in regulated product movement.
Rationale and Implications of the Regulatory Changes
- Administrative Efficiency: Reduces documentation requirements and facilitates faster resolution of import requests.
- Digital Transformation: Supports the transition to electronic platforms in accordance with national digital government policy.
- Regulatory Clarity: Introduces clear, consistent procedures that eliminate redundancies and minimise ambiguity.
- Public Health Assurance: Maintains regulatory oversight while enhancing supply chain agility for essential health and nutrition products.
- Institutional Support: Facilitates expedited imports for public entities procuring health-related goods to address supply demands or urgent public health risks.
The newly gazetted regulation outlines specific implementation timelines depending on the procedure channel, with different effective periods established for digital submissions through the Mexican Foreign Trade Digital Window and for in-person processes related to personal-use imports and public sector acquisitions. This regulatory action marks a significant step forward in the Mexican government?s efforts to modernise public administration, reduce bureaucratic obstacles, and enhance regulatory efficiency within the health sector. COFEPRIS reaffirms its dedication to protecting public health while facilitating more streamlined and accessible regulatory procedures.
Reach out to us if you are interested in exploring opportunities for your products in Mexico and across Latin America.
Published: August 18, 2025
© All rights reserved. All news and diagrams placed on this Web site is made for internal use. Its reproduction or distribution in any form are welcome in case of placing a direct hyperlink to a source. Reproduction or distribution of information which contains FJS International Solutions® as a source is prohibited without the written permission from the FJS International Solutions®. Photos placed on this site are taken from open sources only or purchased.